Diet or Drugs 2? The Eating-Less Question.
Ozempic and Wegovy will curb your appetite. Is that why you lose weight?
When he dieted, he “would get crazy hungry,” Dr. [John] Buse said. With Wegovy, he said, his weight dropped effortlessly until he reached his goal. Then his appetite returned, which was scary. But instead of regaining pounds, he maintained a consistent weight as he continued to take the drug. “The Physicians Really Are Healing Themselves, With Ozempic,” New York Times, February 10, 2025.
Dr. Buse’s story was told in the New York Times article that I discussed in my previous post. He used to get crazy hungry when he tried to calorie-restrict. Most of us do. We eat less than we’d prefer. We spend the day hungry. We obsess about eating.1
But the drugs solve that problem. Once Buse started on Wegovy, all was good; he ate considerably less, his weight dropped, and all without hunger. Apparently, he experienced none of the typical metabolic compensations that associate with weight loss and calorie-restricted diets—not just the hunger and the constant thoughts of food, but the lack of energy as well.
The return of his appetite made Buse understandably anxious, but he’s retained his weight loss. He’s still lean, and seemingly healthier than he’s ever been.
Simple questions: can we learn anything from this? Does it tell us anything meaningful about how these drugs might be working?
I think so, but first… let’s update where we stand with these GLP-1 (glucagon-like-peptide-1) drugs—Wegovy, Ozempic, Mounjaro and their ilk—and this pharmaceutical weight-loss revolution.
As of last May, the latest survey I could find, some 15 million Americans had tried these drugs, maybe 6 million just to lose weight. I’d bet both numbers have gone way up since then.
The drugs in the pipeline seem so potent that they can cause too much weight loss, too quickly—“How much weight loss is too much? Pharma is pushing the limit with new obesity drugs,” as a recent STAT headline put it.
Fat loss is accompanied by significant loss of muscle mass, more than would be expected from dieting alone-- up to 40% of the total weight loss compared to at most 30% with calorie restriction. The authorities can’t agree on whether this loss of muscle mass is a problem, whether it’s adaptive or maladaptive, in the technical lingo. (See here, here and here for a debate.)
And we’re closing in on a world where these drugs are prescribed for prevention of chronic diseases like Alzheimer’s, as the Times recently reported, as is already the case with heart disease.
Considering how fast this field is moving, it’s not surprising, as the Times said, that researchers are “still trying to understand how these drugs impact the brain overall.”
Even in the best of scientific worlds, elucidating the mechanism of a drug’s action—whether in the body or brain—can be an excruciatingly difficult problem to solve. Researchers observe two things happening at once, two concurrent phenomena, and they will assume one causes the other based on their assumptions going in. If those assumptions are right, then that’s reason to have confidence in the interpretations of drug-induced cause and effect.
But what if those initial assumptions are wrong, as assumptions all too often are?
This brings me back to Buse’s experience and the existence of two concurrent phenomena induced by the GLP-1 drugs: significant weight loss and a powerful inhibition of appetite. On these drugs, we’re not hungry: we get full on less food and we stay satiated longer. Result: we eat less. And we lose weight because we eat less.
Here’s how Scientific American put it, quoting Matthew Hayes, a University of Pennsylvania nutritional neuroscientist:
“The way in which GLP-1 drugs are causing weight loss is without question due to suppression of food intake—to satiety,” Hayes adds. Increased satiety means people eat smaller, less frequent meals. In short, they’re a satiety signal.2
I get antsy whenever anyone, scientist or not, says “without question” about a less-than-rigorously-tested hypothesis, but this one seems to make such perfect sense. Can we give Hayes a pass? Well, if you believe that fat storage is determined by how much we eat, then it’s tempting.
Regrettably, I don’t. My bias is that however these drugs are working to generate significant weight loss and inhibiting hunger—the two concurrent phenomena—it’s not the latter that’s causing the former. Indeed, it might be the other way around.
If obesity is not caused by eating too much…
As I’ve written in my books, once the obesity research community in the 1940s fully embraced this idea that we get fat because we eat too much, because we consume more calories than we expend—a conception known as the energy balance hypothesis—they almost religiously ignored the considerable evidence that obesity is a neural-hormonal disorder.
By World War 2, physiologists and biochemists had learned an enormous amount about the hormonal and neural regulation of fat storage and metabolism. Obesity research back then, however, was almost exclusively a European endeavor, dominated by German and Austrian scientists. That research community evaporated with the second World War and the lingua franca of medical science shifted from German to English.
After the war, the field was recreated by young Americans, mostly physicians, who didn’t think twice before embracing the notion that people get fat because they eat too much. As a result, they considered the science of fat metabolism and storage itself to be irrelevant to what they were studying. (Here’s an essay I wrote for STAT that describes this history and its implications.)
If neural and hormonal signals determine whether we use fat to generate energy or store it, perhaps to excess, in our fat cells—a phenomenon known technically as fuel partitioning--then it’s quite possible our appetite and hunger, even our food cravings, might be a response to this fuel partitioning process, and whether or not we’re storing fat or burning it for fuel.
An easy way to conceive of this concept is that we don’t get fat because of how much we eat, but because of what our bodies do with what we eat. Some of us store calories as excess fat and that makes us hungrier, calorie for calorie, than those whose bodies prioritize energy production over storage.
If you’ve got the patience (and you’re a glutton for technical language), you can read about this thinking and the copious evidence supporting it in a comprehensive review that my colleagues and I (led by Mark Friedman) published last year in Obesity Reviews. That the hypothesis is taken seriously—in the diet-related version, the carbohydrate-insulin model--is evidenced by the influential co-authors on this paper published also last summer in Nature Metabolism. Many of my posts will be discussing the evidence for and against these competing hypotheses.
Now let’s get back to Buse. I haven’t forgotten him. An obvious question to ask about his experience is why did his hunger return when his weight plateaued, and why has he been able to maintain his weight loss despite now having to live with his hunger. After all the drugs are supposed to achieve weight loss by making people less hungry. Now we have weight loss maintenance with hunger restored.
Maybe there’s something else going on.
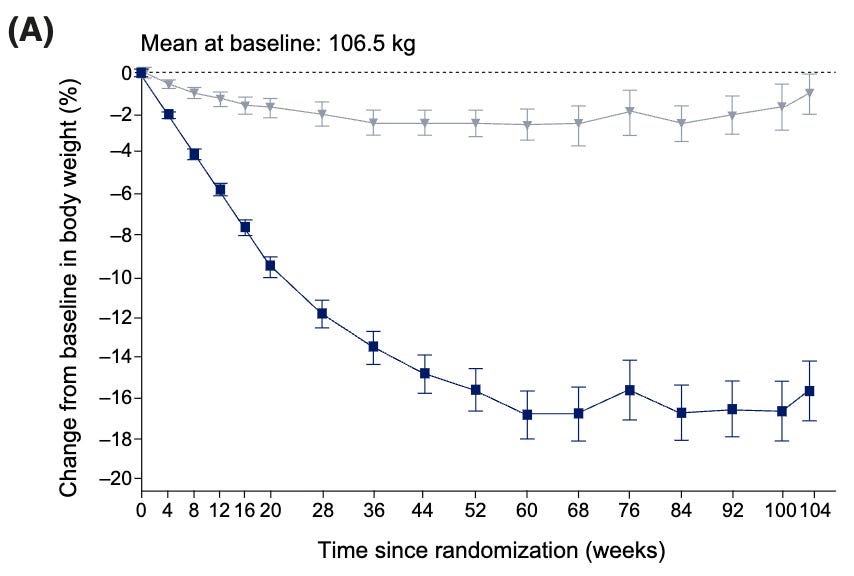
Worth knowing is that what Buse experienced may be the norm. Here it is being reported in the STEP 5 trial, a 2-year study of semaglutide (the active ingredient in Wegovy and Ozempic). The researchers gave the participants the drug or a placebo, monitored their weights, and then asked about their hunger, satiety and food cravings after 20 weeks, 52 weeks and 104 weeks.
Weight loss on the drugs began plateauing at about the one-year mark, maybe a little after (by 60 weeks).
But by the one-year mark, the subjects taking the drug reported sensations of hunger and satiety (fullness) not all that different from those on the placebo. Just as Buse experienced, their hunger apparently came back. By two years, food cravings had returned as well. Like Buse, though, they could continue to control their eating such that they could maintain their weight loss.
If they can lose 50 pounds and only be as hungry after the weight loss as they were before, maybe they’ll only eat as much food as they need, and not too much. That their weight remains stable, even as their hunger returns, suggests this is happening.
But that’s a phenomenon that’s hard to explain if you think that the drugs work in the brain by getting people to eat less.
Now we have to assume that the brain somehow knows how much these people should ideally weigh—a concept called the set point, which has been debated and contested for the 60 years since obesity researchers started taking it seriously—or should weigh on the drug, and that when they reach that weight, the brain responds by unfettering hunger. The assumption is that if they start gaining weight, the hunger will go away again and all will be good.
When I discussed this with one influential physician delighted with his Wegovy experience, he said, “My brain/body wants me to weigh ~160-165 pounds while taking Wegovy 2.4 mg as opposed to 210-220.”
Maybe.
Here’s another possibility: What if our experience of hunger and satiety is dependent not on how much our brains want us to weigh—this set point idea—but whether cells elsewhere in the body are generating enough energy to run comfortably? That, in turn, will depend on how accessible the energy is in the fat tissue, whether it can be mobilized readily when needed, and whether or not the cells in our lean tissue, our muscles and organs, will use it for fuel.
These metabolic phenomena are determined by hormonal and neurological signals more so than anything else.
A relationship between hunger and fat metabolism?
This concept emerged in the 1970s out of the field of physiological psychology. The researchers doing this work were focused on understanding hunger, appetite and other phenomena, like thirst, that should seemingly be responses to underlying physiological states. The hypothesis that emerged from this perspective was never refuted, only, for the most part, ignored by the obesity researchers. (Here’s a lengthy 2021 review of this physiological perspective on hunger by USC and ETH-Zürich researchers.)
By this alternative thinking, there’s no set point responding to all these myriad (perhaps infinite?) signals drifting up from the body, such that our brains can compute whether or not are fat stores going up or down and adjust intake (hunger/appetite) and expenditure accordingly to keep them stable.
Rather our brains care that the cells in the rest of the body are being properly fed—that’s what’s important, after all—and that’s what they’re monitoring: are the cells in the periphery (everything below the neck) generating energy at a rate that suggests they have plenty of energy available.3 By this thinking, our brains attend (via the vegus nerve and other signals) to the production of energy by liver cells—ATP, specifically, for those who remember their high school biology. (And perhaps directly to a molecule called AMPk, which is created when ATP is depleted.)4
The liver can be thought of as a central clearing house for all the fuels-- proteins, fats, and carbohydrates--we consume and mobilize from storage. These fuels all pass through the liver, whether they’re coming from the food we eat and our guts or the food we’ve stored in fat tissue or muscles (protein). The liver determines what we do with these fuels: how they’re partitioned, in effect, between use for energy, for repair of other cells, or further storage. So monitoring the liver specifically for its energy status makes a lot of sense.
By this thinking, feed the liver, and the brain is content. The liver acts, in effect, as the sense organ of fuel availability in the body. Shift fuels away from the liver, into storage, growth, physical activity, or even reproduction, and the brain detects the decrease in ATP generation and responds with hunger and food seeking behavior. “A shift in fuel partitioning toward synthesis, storage and sequestration of fat,” as we phrased it in our Obesity Reviews paper, “results in the increase in food intake usually associated with the development of obesity.”
So what does this have to do with the GLP-1 drugs? Well, the opposite would also be true. Shift fuel partitioning away from “synthesis, storage and sequestration of fat” and toward energy production in the cells, and specifically the liver, and the result would be a decrease in food intake.
If the GLP-1 drugs cause this shift in partitioning, if that’s their mechanism of action, then people who don’t feel hunger on these drugs wouldn’t feel it because their cells are readily burning the fat from their fat tissue and so generating ATP as they should. The liver cells aren’t hungry, and so neither are we, because the liver cells are being fed. The absence of hunger and eating less is a result of losing fat and burning that fat for fuel, not a cause of weight loss.
(The same would be true of diets, which is why folks like me think successful weight loss diets, those that allow weight loss without hunger and a significant reduction in energy expenditure, do so not by making people eat less, but by changing the hormonal milieu in their bodies—minimizing insulin secretion, per the carbohydrate-insulin model—such that fat is readily released from fat cells and used by the rest of the body for fuel.)
Here's an easy way to think about it: imagine you take a drug (a GLP-1 drug like Wegovy, say) that allows you to lose two pounds of fat a week. That’s two pounds of fat every week that is being mobilized from your fat tissue, released into the circulation as fatty acids, and then used by your cells to generate energy. Convert that into calories, and it’s 7000 calories of fat you’d be using to generate energy every week, 1000 calories every day, that you would not be using if you were weight stable. That’s the equivalents of the calories in 1 ¼ sticks of butter every day. As far as your cells and your brain are concerned, it’s no different than eating 1 ¼ sticks of butter every day. Now if you’re not particularly hungry, it shouldn’t be that difficult to imagine why.
Moreover, this inhibition of hunger would continue until you became weight stable again, at which point your hunger would return because you’d lose access to that ready supply of excess fat. When weight loss plateaus, hunger returns because of it. Not the other way around, not by this way of thinking,
This may or may not be right, but what Buse experienced--the reemergence of hunger with weight plateauing--and what the subjects in STEP 5 experienced is what would be expected from this fuel-partitioning perspective.
Given our two concurrent phenomena caused by the drugs—weight loss and inhibition of appetite—how would we test which is the primary effect?
One obvious way would be to control for how much people eat on these drugs and see what happens. If we can remove the effect of appetite inhibition, do we still see differences in weight loss, fat used for energy, and total energy expenditure? If so, then maybe the appetite inhibition is a result of the effect of these drugs on fat storage and metabolism, as the fuel-partitioning hypothesis would have it.
Ideally, researchers give one group of subjects the drug, see how much they eat every day, and then give that same amount of food to a control group that’s getting a placebo. This is a technique called pair-feeding, and it’s far easier to do with rats and mice in a lab than people. When researchers have done these studies in mice and rodents, as we detailed in our Obesity Reviews article, they’ve inevitably (ok, maybe one exception) supported the fuel-partitioning perspective on weight control.
The closest we have for the GLP-1 drugs is a single study published in 2023 by a team of researchers, two of whom (Kevin Hall and Eric Ravussin) were co-authors on the article I mentioned above about this alternative fuel-partitioning hypothesis being taken seriously.
This was only a 19-day study and a preliminary one at that (i.e., I wouldn’t want to rely on it in a court of science). The researchers randomized subjects into a group taking an experimental GLP-1 drug and another getting a placebo, They then fed both groups diets with 1000 calories/day fewer than they needed, in theory, to maintain a stable weight.5
What they observed was precisely what the fuel-partitioning theory would have predicted: those treated with the drug lost more weight, fat mass and fat free mass, despite eating (roughly) the same amount of calories. Their energy expenditure went down less than it did in the placebo group, and their bodies burned more fat (technically, they oxidized more fat), and their livers synthesized and secreted more ketones.
The difference in fat oxidation and ketogenesis suggest that the drug worked to get more fat out of the fat tissue, such that it could be metabolized in the liver, and the liver was doing just that.
This shouldn’t have been unexpected because there is one other study in the literature,6 published back in 2006, in which NIH researchers tried to assess the effect of the GLP-1 our bodies produce naturally on the fuel supply preferentially used by the liver. They reported that the greater the level of fasting GLP-1, the greater the overall energy expenditure and the more fat is oxidized.
The researchers thought this was great, they said, because these metabolic effects went “along with the established role of GLP-1 in decreasing appetite and promoting satiety in both animal models and humans.” Hence, even better should GLP-1 drugs be tried for obesity someday, which of course is what’s happened. What the researchers didn’t consider, because they were unaware it was a possibility, was that the effect of GLP-1 on fat oxidation and energy expenditure could be causing those “established” effects on appetite and satiety. If you’ve only got one working hypothesis—obesity, in this case, is caused by eating too much—you interpret all your evidence from only that perspective.
But these drugs do work in the brain! Yes, but…
One last note: I have to give the GLP-1 researchers credit here, at least for a couple of paragraphs.
They also assume the drugs work in the brain because they have rodent experiments that establish this pretty close to unambiguously. The study that’s invariably sited was published in 2014. The researchers, led by Randy Seeley, then of U. Cincinnati, created mice that lacked the receptors for the GLP-1 drugs either in the brain—the central nervous system (CNS)—or on the peripheral nerves.
The mice that lacked the CNS receptors failed to lose weight when given a GLP-1 drug. So the brain is key. If the drugs don’t work in the brain, then they don’t cause weight loss. Researchers since then have interpreted this finding as evidence, if not proof, that the drugs’ primary mechanism of action is appetite inhibition. What else could the drug be doing in the brain, after all?
This is one of the aspects of obesity-related research that still mystifies me after all these years. We’ve known for 150 years that the brain and the central nervous system regulate fuel metabolism peripherally, ever since the legendary French physiologist Claude Bernard used a needle to puncture the fourth ventricle of a dog’s brain and created what looked like diabetes. He then showed this could be prevented by severing the nerves from brain to liver.
An enormous amount of research has been done since then on how the brain, via the central nervous system, regulates fuel storage, synthesis and oxidation. Here’s a 2014 review of how the CNS works to get fat out of fat tissue, and a review of how it works to metabolize that fat once it does.
But once the obesity researchers convinced themselves that we get fat because we eat too much, that gave them tunnel vision. They interpret their observations through that perspective exclusively. If something works in the brain to cause fat accumulation or fat loss, as the drugs do, they assume it must work by making people eat too much or allowing them to eat less. Evidence to the contrary gets a raised eyebrow before being swept under the cognitive rug. It’s never allowed to accumulate to the point that assumptions have to be rethought.
All this can be tested further, of course, but the obesity researchers have to care enough to do it.
This is a phenomenon that’s now known as “food noise”, although there’s nothing new about it. Semi-starve anyone, fat or lean, as the U. Minn nutritionist Ancel Keys did back in the 1940s in a famous experiment, and they’ll think about food constantly, too.
The effect on satiety—how quickly you feel full and for how long after the meal ends—is generally assumed to be mediated, at least in part, through the drugs’ effect on gastric emptying. The food takes longer to get out of the gut and into the circulation, and this generates a new set of neural-hormonal consequences. A subject for another post.
If you were going to write a popular book about this thinking, it would not be called The Hungry Brain or The Hungry Gene, but perhaps The Hungry Cell.
Here’s a not-that-technical review of this liver-centric view of feeding behavior by my colleague Mark Friedman.
“In theory,” because this calculation depends on which hypothesis of obesity is correct—the fuel-partitioning or the energy-balance hypothesis—and its implications.
To be accurate, I could only find one other study. If anyone can find others, please send along links and I’m happy to stand corrected as necessary.






Excellent and I can give you another example. My nurse practitioner is a 5 foot four bodybuilder competitor. She weighs may be 120 pounds of solid muscle. She used tirzepatide to get ready for competition to lose a few extra pounds. She is meticulous eating the exact amount of food every day measured and quantified. She also is meticulous about energy expenditure. She told me she was convinced that the drug facilitated fat metabolism independent of food intake. In other words, she lost the fat exactly as you said someone turned on her lipoprotein lipase - she selectively can mobilize fat. I will have to share this with her because we've had a long discussion and she was absolutely convinced it was a direct effect on fat metabolism and not calories. She said exactly like you outlined. The drug takes the fat off, keeping everything the same.
Very interesting.
One other point I've been talking to patients about this at length . I've been on 5 mg of Tirzep. Now for two years and have maintained ideal weight. The big mistake people are making is that they're in a big hurry to lose weight. What they do is they ramp up the dose at four weeks eight weeks. I think it's a big mistake. They end up getting more side effects and it's not tolerated as well. There's no rush. The brain takes time as does the body to change and I told people give it eight weeks 12 weeks even before you go up on the dose. People have done that and they found that they can be stable on lower doses, which is cheaper and have less of the G.I. side effects. Great reporting as always.
I’m curious whether you have any thoughts on how the GLP-1-associated fuel partitioning process detects that “enough” weight has been lost.
If the drugs are just an on-switch for mobilizing fats for fuel, we would expect that process to continue until the body becomes emaciated, or expires. Since this isn’t happening (hunger returns at 10-25% body fat, not 0% body fat), I’m curious what mechanisms have been proposed for the switch getting flipped off again.
Worth noting in this discussion, in my experience with LCHF/keto diets, there is also a point where weight loss appears to reach a “natural” limit, but in contrast to what is being reported with the drugs, hunger does not return. So what do the differences between hunger at maintenance on GLP-1 drugs vs keto diets help us understand about these interventions?